Abstract
Background: Cord blood (CB) transplantation (CBT) has several advantages compared with other graft sources. CBT has low rates of chronic graft-versus-host disease (GVHD) and allows for several human leukocyte antigen (HLA) mismatches, thus expanding donor availability. However, CBT has fallen into disfavor because of its low cell dose and associated prolonged cytopenias, which result in prolonged hospitalization and higher risk of non-relapse mortality (NRM). Furthermore, the need for large CBs leads to selection of poorly HLA matched CBs, further increasing NRM.
UM171 is a small molecule that expands hematopoietic stem cells. From 2016 to 2018, 22 patients with high-risk malignancies who lacked a donor were transplanted on a phase I-II clinical trial with a single UM171-expanded CB (Cohen S et al. Lancet Haematol 2019) after a myeloablative conditioning. The goal was to accelerate engraftment while selecting smaller, better HLA matched CBs to decrease NRM while preserving the benefits of CBT. Results demonstrated that indeed UM171-expanded CB has the potential to overcome slow neutrophil engraftment and diminish NRM. The aim of the current analysis was to examine outcomes after conventional single/double ≥ 4/6 HLA-matched CB and 8/8-matched unrelated donor (MUD) peripheral blood stem cell (PBSC) transplantation with UM171-expanded CBT.
Methods: Data on UM171-expanded adult CBT recipients were drawn from the phase I-II trial. Data for adult recipients of myeloablative CB and PBSC transplants for hematologic malignancies (acute myeloid and lymphoblastic leukemia, chronic myeloid leukemia, non-Hodgkin and Hodgkin lymphoma) in the United States and Canada during 2014-2019 with a Karnofsky performance status (KPS) score ≥70 were obtained from the Center for International Blood and Marrow Transplant Research (CIBMTR) database. CB recipients who received anti-thymocyte globulin or alemtuzumab in the conditioning were excluded from the CIBMTR population. Patients were directly matched for the number of prior allogeneic transplants (alloHCT), refined disease risk index (for 1st alloHCT), disease status (for 2nd alloHCT), and acute myeloid leukemia cytogenetic risk. Patients were matched by propensity score for age, comorbidity index and KPS. CIBMTR attempted to identify 4 controls per UM171 patient. Clinical outcomes included engraftment, NRM, progression-free survival (PFS), overall survival (OS), relapse, GVHD-free, relapse-free survival (GRFS), acute GVHD grade III-IV, and chronic GVHD. PFS, OS, and GRFS were estimated by Kaplan-Meir method; other outcomes were calculated using cumulative incidence estimates; marginal Cox regression models were used for analyzing NRM and GRFS.
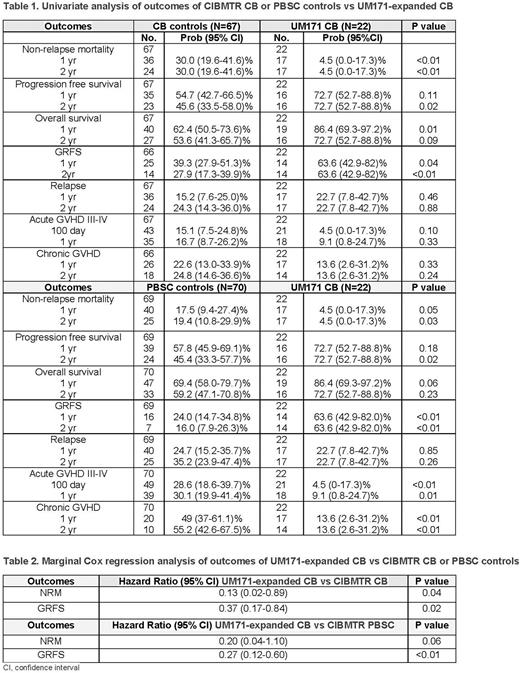
Results: Overall, 137 CIBMTR (67 CB, 70 PBSC) and 22 UM171-expanded CBT patients participated. Univariate analysis and marginal Cox regression analysis results are shown in Tables 1 and 2, respectively. Median time to neutrophil engraftment was 18 days (range 10-30) for UM171-expanded CB recipients compared to 21 days (range 8-53) for CIBMTR CB recipients (p<0.01) (data not shown). Univariate analysis revealed less 1- and 2-year NRM, improved 2-year PFS, improved 1-year OS, and 1- and 2-year GRFS for UM171-expanded CBT compared to CIBMTR CB controls. The UM171 cases had less 1- and 2-year NRM, improved 2-year PFS, and improved 1- and 2-year GRFS compared to CIBMTR PBSC controls. Furthermore, UM171-expanded CB patients experienced less acute GVHD III-IV and chronic GVHD compared to PBSC recipients. In marginal Cox regression analysis, UM171-expanded CBT recipients were 87% and 63% less likely than CIBMTR CB controls to experience NRM and GRFS events, respectively, and 73% less likely to experience GRFS events than CIBMTR PBSC recipients.
Conclusions: This real-word evidence suggests that UM171-expanded CB recipients have select improved outcomes compared to conventional CB and MUD PBSC recipients. While this study was limited by the small number of UM171 CB trial participants and inability to identify the planned number of controls for each case, UM171 expansion of CB appeared to achieve the desired outcome of maintaining CB's benefits while removing its disadvantages. Thus, prospective randomized studies of UM171-expanded CBT compared to MUD PBSC alloHCT are warranted to confirm these results.
Study Support: This study was funded by ExCellThera.
Disclosures
Cohen:ExCellThera: Consultancy, Patents & Royalties, Research Funding. Bambace:AbbVie: Consultancy; Bristol Myers Squibb: Consultancy. Burns:Astellas: Research Funding; BMS: Research Funding; Gamida Cell: Research Funding. Roy:ExCellThera: Patents & Royalties. Roy:Pfizer: Speakers Bureau; Knight Therapeutics: Consultancy; Kiadis Pharma: Consultancy, Honoraria. Veilleux:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite-Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees. Zhang:Gamida Cell: Research Funding; Bristol Myers Squibb: Research Funding; Astellas Pharma Inc: Research Funding. Tang:Kite Pharma: Other: Spouse is currently employed at Kite; Bristol Myers Squibb: Research Funding; Gamida-Cell Ltd: Research Funding. Sauvageau:BMS: Research Funding; ExCellThera: Consultancy, Current Employment, Current equity holder in private company, Honoraria, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.